A System Receives 575 J Of Heat
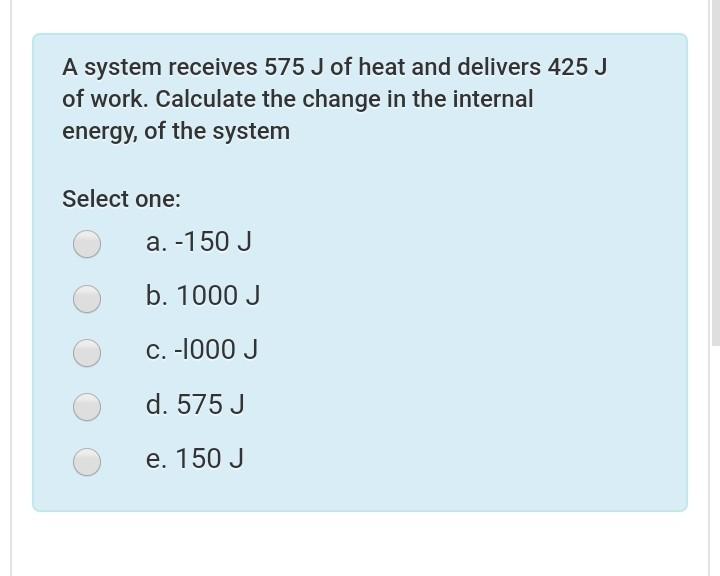
A system receives 575 j of heat. Calculate the change in the internal energy ΔE of the system. Calculate the change in the internal energy E of the system. Calculate the change in the internal energy ΔE of the system.
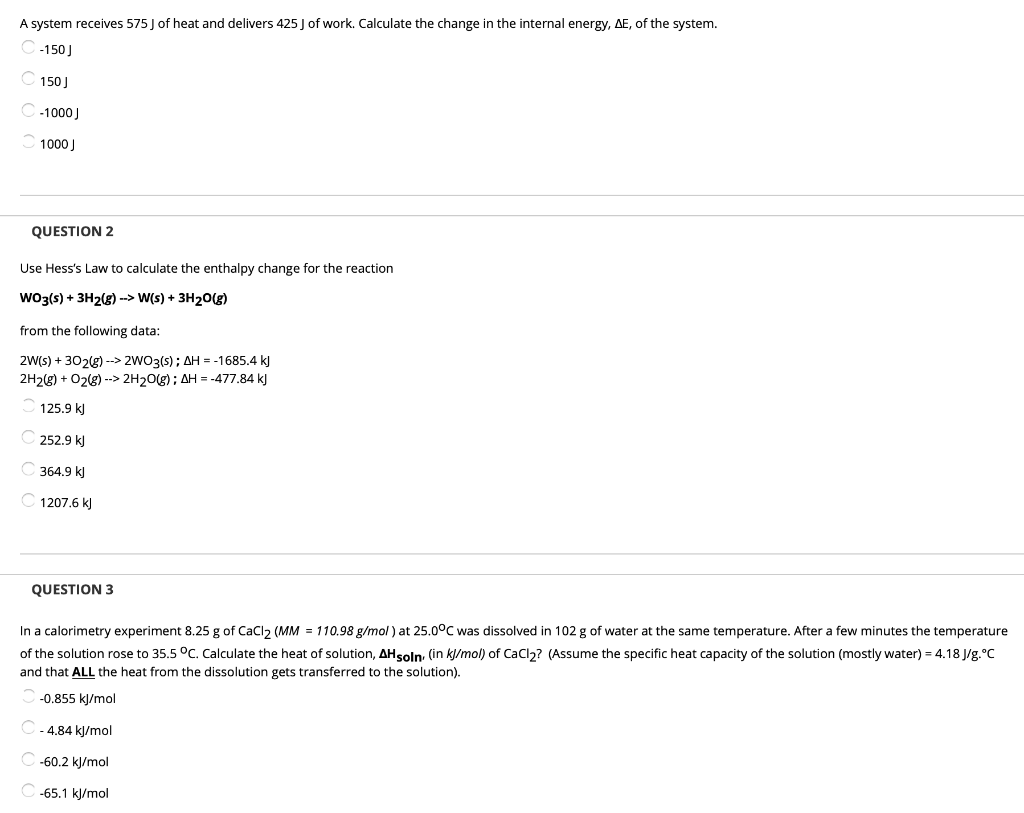
Asked Aug 22 2019 in Chemistry by fishnut88. A -150 J B 150 J C -l000 J D 1000 J E 575 J. ΔE 575 -425 ΔE 575 - 425.
E 575 J B. A system receives 575 J of heat and delivers 425 J of work. A system receives 575 J of heat and delivers 425 J of work.
Q is total amount of heat energy going in or coming out. A system receives 575 J of heat and delivers 425 J of work. A system receives 575 J of heat and delivers 425 J of work.
A-150 J b 150 J c-1000 J d 1000 J e 575 J. Solution for A system receives 575 J of heat and delivers 425 J of work. A system receives 575 J of heat and delivers 425 J of work.
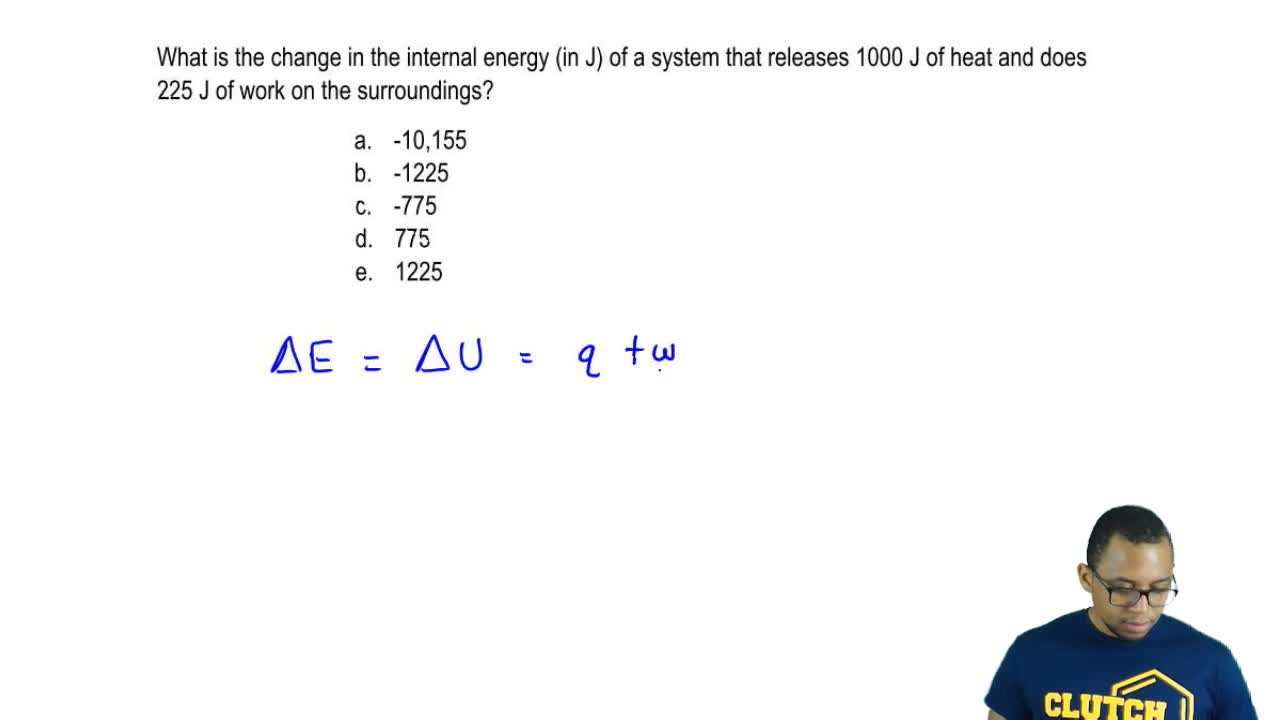
88 A system delivers 225 J of heat to the surroundings while delivering 645 J of work. Calculate the change in the internal energy ΔE of the system. A system receives 575 J of heat and delivers 425 J of work.
A gas in a cylinder was placed in a heater and gained. A system receives 575 J of heat and delivers 425 J of work.
A system receives 575 J of heat and delivers 425 J of work.
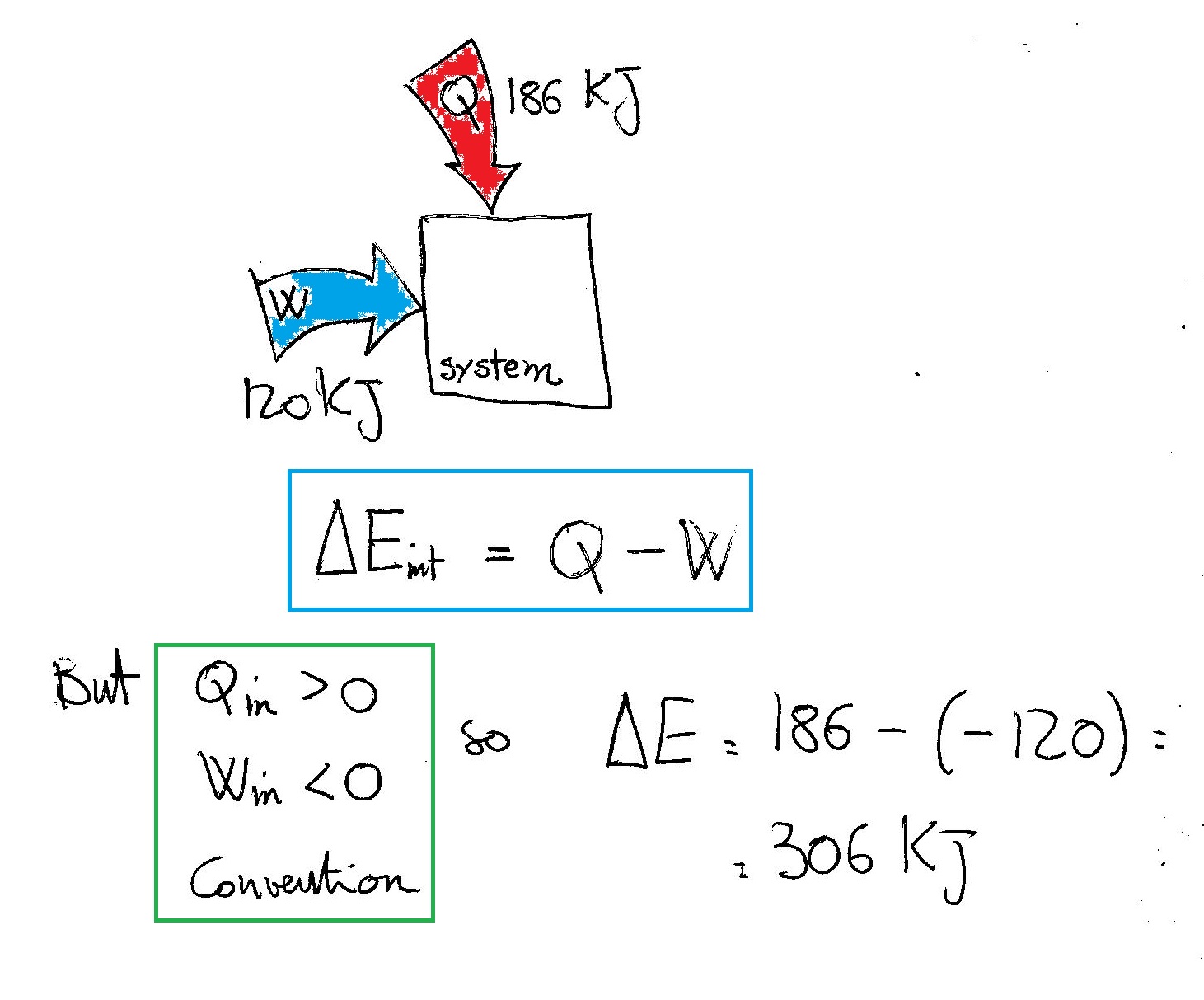
Also we are told that the system delivered 425 J of work. A -150 J B 150 J C -1000 J D 1000 J E 575 J. The internal energy ΔE or ΔU of the system can be calculated from the heat q and work w of the system where. A system receives 575 J of heat and delivers 424 J of work. Thus w -425 J since work was expended. A system receives 575 J of heat and delivers 425 J of work. Also we are told that the system delivered 425 J of work. Calculate the change in the internal energy ΔE of the system. Calculate the change in the internal energy of the system Select one.
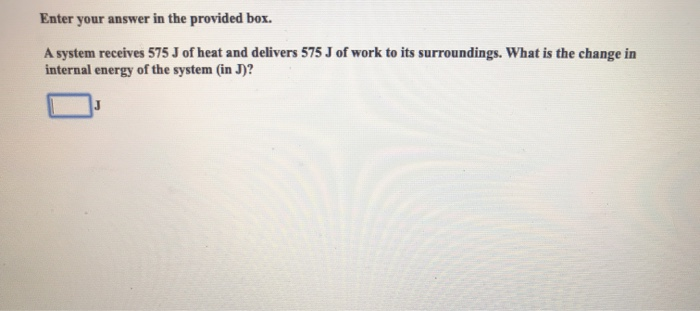
A system receives 575 J of heat and delivers 425 J of work. Delta E ΔE of the system. Calc the change in the internal energy. A system receives 575 J of heat and delivers 424 J of work. Calculate the change in the internal energy of the system Select one. What is the change in the internal energy ΔU of the system. A-150 J b 150 J c-1000 J d 1000 J e 575 J.




































Post a Comment for "A System Receives 575 J Of Heat"